Latest Updates
-
 Holi 2026: 12 Incredible Ways Different States Celebrate the Festival of Colours
Holi 2026: 12 Incredible Ways Different States Celebrate the Festival of Colours -
 Lunar Eclipse 2026: Health, Food, And Pregnancy Myths Debunked
Lunar Eclipse 2026: Health, Food, And Pregnancy Myths Debunked -
 Holi 2026: Are ‘Herbal’ Holi Colours Really Safer? Expert Explains The Safety Gap
Holi 2026: Are ‘Herbal’ Holi Colours Really Safer? Expert Explains The Safety Gap -
 Horoscope for Today March 03, 2026 - Small Choices, Steady Progress
Horoscope for Today March 03, 2026 - Small Choices, Steady Progress -
 Holi Dos and Don’ts 2026: Astrologer’s Tips For A Positive And Prosperous Year
Holi Dos and Don’ts 2026: Astrologer’s Tips For A Positive And Prosperous Year -
 Holi Muhurat Guide 2026 According To Astrologer: Dates, Ritual Timing, And Lunar Significance
Holi Muhurat Guide 2026 According To Astrologer: Dates, Ritual Timing, And Lunar Significance -
 Holi and Kids: Essential Do’s and Don’ts for Protecting Delicate Skin During the Festival of Colours
Holi and Kids: Essential Do’s and Don’ts for Protecting Delicate Skin During the Festival of Colours -
 Chandra Grahan 2026: Sutak Kaal, Religious Significance and Timings in India
Chandra Grahan 2026: Sutak Kaal, Religious Significance and Timings in India -
 Total Lunar Eclipse 2026: Timings, Where To Watch, and Visibility in India
Total Lunar Eclipse 2026: Timings, Where To Watch, and Visibility in India -
 Happy Holi 2026: 50+ Ready-to-Share Messages for Family, Friends, Partner, WhatsApp & Instagram
Happy Holi 2026: 50+ Ready-to-Share Messages for Family, Friends, Partner, WhatsApp & Instagram
Biologics: What Are They & How They Help Treat Psoriasis
Psoriasis is a common skin condition that speeds up the life cycle of skin cells. The condition causes the rapid build-up of cells on the surface of the skin, where it forms scales and red patches that are itchy and sometimes painful. The chronic autoimmune condition is the result of a sped-up skin production process.

Scales typically develop on joints, such elbows and knees and less common types of psoriasis affect the nails, the mouth, and the area around genitals. Psoriasis is classified into five different types and they are plaque psoriasis, guttate psoriasis, pustular psoriasis, inverse psoriasis and erythrodermic psoriasis [1] [2] .
The common treatment options for psoriasis are topical treatments, light therapy and systemic medications. However, studies have pointed out that the traditional treatment methods may not be effective for everyone and one will have to try out many different treatments before finding the right and effective one[3] .
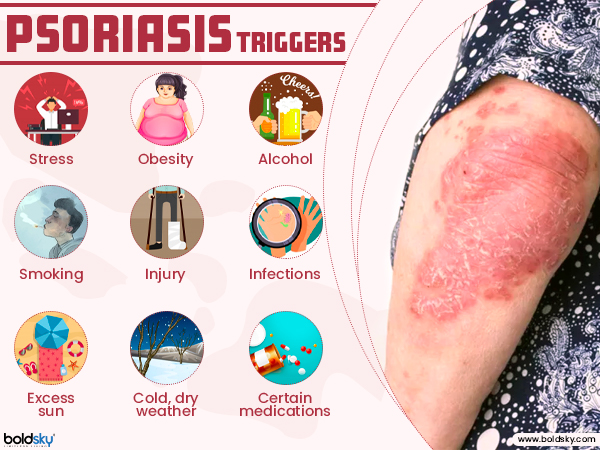
The current article will focus on exploring the role biologics play in the treatment of psoriasis.
What Are Biologics?
Biologics or biologic drugs are powerful medications which are made from components like sugars, proteins, or DNA or can be whole cells or tissues. They are also made from all sorts of living sources such as mammals, birds, insects, plants, and even bacteria.
Biologics are different from traditional systemic drugs that impact the entire immune system as they only target specific parts of the immune system. These are at the forefront of medical advances today and are genetically changed in the lab to make certain proteins [4] .
Biologic drugs are given by injection (shot) or intravenous (IV) infusion (a slow drip of medicine into your vein). Biologics treat a wide range of common and rare diseases and include vaccines, cell and gene therapies, tissues for transplants, and more.
Some of the most common types of biologics are as follows [5] :
- Insulin glargine: It is a long-acting form of human insulin that is used once daily to control blood sugar levels in individuals with diabetes.
- Adalimumab: These are one of the most commonly used biologics and is used for the treatment of rheumatoid arthritis (RA) and other autoimmune diseases like psoriasis and Crohn's disease.
- Trastuzumab: One of the most widely used biologics for cancer, this one has been proven effective in treating breast cancer and stomach cancer.
- Bevacizumab: This one is used for treating patients with retinal diseases like age-related macular degeneration.
- Onabotulinumtoxina: Also known as botox, this one is produced by a bacteria called Clostridium botulinum. Commonly used in cosmetic surgeries, Botox is also beneficial for the treatment of various disease and conditions.
Currently, biologics have a significant place in the medical field due to its ability to treat certain illnesses where no other treatments were available. However, due to its diverse sources, biologics are usually much more complicated than other drugs because they take a lot more work to purify, process, and produce [6] [7] .
And once the biologics are developed, they tend to be more unpredictable and are often more susceptible to light and temperature [8] .
Biologics For Psoriasis
When all the other common treatment methods fail, doctors prescribe biologics for the treatment of psoriasis. People with mild to moderate psoriasis can usually manage their disease well with topical treatments [9] . However, it is not the same in the case of individuals with severe or even moderate cases of psoriasis. One of the major problems reported in the case of the traditional psoriasis treatment is the treatments losing effectiveness over time [10] .
More and more people are opting for biologics in the treatment of psoriasis. These genetically engineered treatment methods do not cure psoriasis but help treat the symptoms. Biologics are effective in releiving the symptoms because they target a specific part of the immune system that is involved in causing psoriasis [11] [12] .
In comparison to other treatment methods for psoriasis, biologics only require fewer doses. Some biologics have to be injected once per week, where some only need to be injected once every 12 weeks after the first two initial doses [13] .
One of the other major reasons for the increasing demand of biologics for psoriasis is that the usually used treatments like cyclosporine, corticosteroids, and methotrexate are known to cause side effects like mouth sores, nausea, upset stomach, and even skin cancer. And biologics, they work in a more selective way than other psoriasis treatments by targeting specific proteins in the immune system that have been proven to be associated with psoriasis - and thereby causing fewer side effects in comparison [14] [15] .
Biologics can also be combined with your current treatment methods, which in turn, can improve the efficacy of your treatment and lower the dose - which also aids in decreasing the side effects.
Biologics Cause Less Severe Side Effects
Like any other treatment options, biologics also tend to cause side effects but they tend to be less severe. The most common side effects are as follows [16] :
- Allergic reactions
- Injection site reactions
- Chills
- Weakness
- Diarrhoea
- Nausea
- Vomiting
- Rash
- Itching
- High blood glucose levels
- Cough
- Constipation
In some cases, they have been reported to trigger headaches, muscle pain, insomnia, back pain, abdominal pain, dizziness etc. In severe case, biologics have been reported to cause low blood pressure, serum sickness, bleeding, anaemia etc.
Doctors and studies have asserted that pregnant women and breastfeeding mothers should avoid the use of biologics as it could be harmful to the foetus because of their mechanism [17] . And in the case of breastfeeding mothers - many drugs are excreted in breast milk and there may present a risk of serious adverse effects in the infant- therefore, consult your doctor if you are undergoing biologic treatment for psoriasis [18] .
On A Final Note...
Biologics are not new to the medical world, it was introduced and approved in 2003. However, researchers have gathered quite a bit of evidence in the past decade to support the efficacy as well as the safety of biologics, which indeed has proved to promote its application as a treatment option for various health problems [19] [20] .
You must avoid biologics if your immune system is significantly compromised, you have an active infection, you recently received a live vaccine or flu shot or if you are pregnant or nursing. With research continuing and expanding in the field of biologics, it is more than likely that even more treatment options will be available shortly. Switching psoriasis treatments is a common and accepted practice, consult with your doctor and discuss the applicability of biologics for your psoriasis.
Disclaimer: The information provided in this article is for general informational and educational purposes only and is not intended as a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or a qualified healthcare provider with any questions you may have regarding a medical condition.
- [1] Baurecht, H., Hotze, M., Brand, S., Büning, C., Cormican, P., Corvin, A., ... & Franke, A. (2015). Genome-wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms. The American Journal of Human Genetics, 96(1), 104-120.
- [2] Takeshita, J., Grewal, S., Langan, S. M., Mehta, N. N., Ogdie, A., Van Voorhees, A. S., & Gelfand, J. M. (2017). Psoriasis and comorbid diseases: epidemiology. Journal of the American Academy of Dermatology, 76(3), 377-390.
- [3] Baliwag, J., Barnes, D. H., & Johnston, A. (2015). Cytokines in psoriasis. Cytokine, 73(2), 342-350.
- [4] Docheva, D., Mueller, S. A., Majewski, M., & Evans, C. H. (2015). Biologics for tendon repair. Advanced drug delivery reviews, 84, 222-239.
- [5] Anselmo, A. C., Gokarn, Y., & Mitragotri, S. (2019). Non-invasive delivery strategies for biologics. Nature Reviews Drug Discovery, 18(1), 19-40.
- [6] Kinch, M. S. (2015). An overview of FDA-approved biologics medicines. Drug discovery today, 20(4), 393-398.
- [7] Singh, J. A., Hossain, A., Ghogomu, E. T., Mudano, A. S., Maxwell, L. J., Buchbinder, R., ... & Wells, G. A. (2017). Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews, (3).
- [8] Rider, P., Carmi, Y., & Cohen, I. (2016). Biologics for targeting inflammatory cytokines, clinical uses, and limitations. International journal of cell biology, 2016.
- [9] Dong, J., & Goldenberg, G. (2017). New biologics in psoriasis: an update on IL-23 and IL-17 inhibitors. Cutis, 99(2), 123-127.
- [10] Mahajan, R. (2018). Biologics in Psoriasis. World Clinics: Dermatology: Psoriasis: Volume 2, Number 1, 2(1), 94-117.
- [11] Nishikawa, R., Nagai, H., Bito, T., Ikeda, T., Horikawa, T., Adachi, A., ... & Nishigori, C. (2016). Genetic prediction of the effectiveness of biologics for psoriasis treatment. The Journal of dermatology, 43(11), 1273-1277.
- [12] Honda, H., Umezawa, Y., Kikuchi, S., Yanaba, K., Fukuchi, O., Ito, T., ... & Nakagawa, H. (2017). Switching of biologics in psoriasis: reasons and results. The Journal of dermatology, 44(9), 1015-1019.
- [13] Rencz, F., Kemény, L., Gajdácsi, J. Z., Owczarek, W., Arenberger, P., Tiplica, G. S., ... & Péntek, M. (2015). Use of biologics for psoriasis in Central and Eastern European countries. Journal of the European Academy of Dermatology and Venereology, 29(11), 2222-2230.
- [14] Zweegers, J. (2017). Different perspectives on psoriasis care with biologics: to explore, to compare, and to predict treatment outcome with biologics in psoriasis (Doctoral dissertation, [Sl: sn]).
- [15] Mercieca, L., Lavery, D., Warren, R. B., & Griffiths, C. E. M. (2019). Long‐term, real world, efficacy of biologics for psoriasis: a single centre's experience. British Journal of Dermatology.
- [16] Betegnie, A. L., Gauchet, A., Lehmann, A., Grange, L., Roustit, M., Baudrant, M., ... & Allenet, B. (2016). Why do patients with chronic inflammatory rheumatic diseases discontinue their biologics? An assessment of patients’ adherence using a self-report questionnaire. The Journal of rheumatology, 43(4), 724-730.
- [17] Zweegers, J., van den Reek, J. M. P. A., van de Kerkhof, P. C. M., Otero, M. E., Kuijpers, A. L. A., Koetsier, M. I. A., ... & Njoo, M. D. (2016). Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side‐effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice: a prospective, comparative, long‐term drug‐survival study from the Bio CAPTURE registry. British Journal of Dermatology, 175(2), 340-347.
- [18] Mease, P. J. (2016). Biologic Therapy of Psoriatic Arthritis. In Psoriatic Arthritis and Psoriasis (pp. 295-308). Springer, Cham.
- [19] Rider, P., Carmi, Y., & Cohen, I. (2016). Biologics for targeting inflammatory cytokines, clinical uses, and limitations. International journal of cell biology, 2016.
- [20] Park, S. C., & Jeen, Y. T. (2015). Current and emerging biologics for ulcerative colitis. Gut and liver, 9(1), 18.



 Click it and Unblock the Notifications
Click it and Unblock the Notifications












